Atoms are tiny particles of matter which make up everything in the universe. Everything you see is made up of atoms.
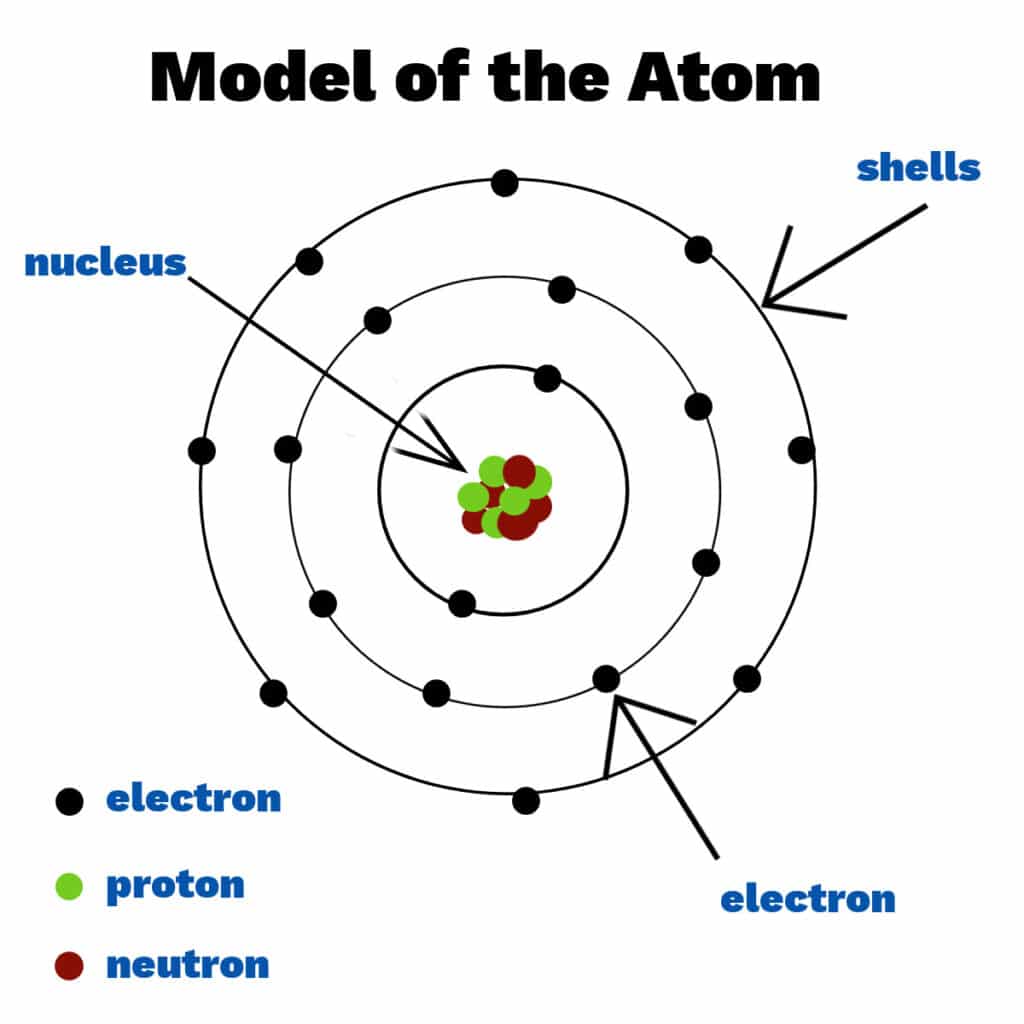
Scientists spent many years trying to understand the structure of the atom, with several models and theories stuff disproved or improved upon withal the way. Today we know that atoms consist of a nucleus containing protons and neutrons, surrounded by shells ( energy levels ) of electrons. Electrons, protons and neutrons are known as subatomic particles. Each shell holds a stock-still number of electrons.
Atoms from variegated elements have variegated numbers of electrons, protons and neutrons.
Theory of Two-bit Structure
John Dalton
John Dalton is often known as the father of two-bit theory. He proposed a theory of the whit in 1803.
John Dalton believed that:
All matter is made of atoms
Atoms were solid spheres – later disproved
Atoms within an element are the same. Atoms from variegated elements are not.
Atoms couldn’t be remoter wrenched lanugo – later disproved
Atoms are rearranged during a chemical reaction but are not lost. This is the Law of Conservation of Mass.
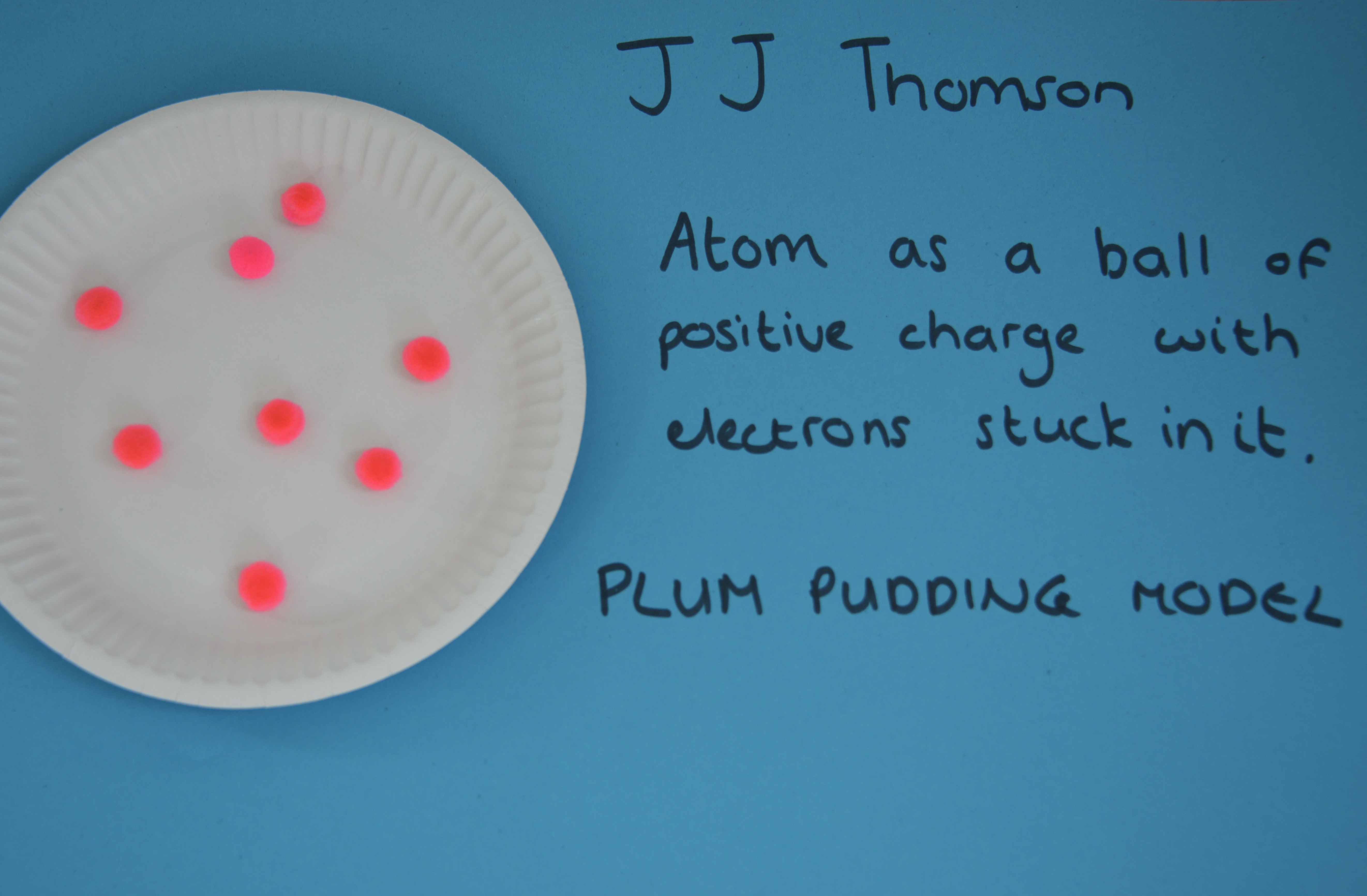
JJ Thomson
In 1897, JJ Thomson proposed that atoms were not solid spheres. His research showed that atoms must contain negatively charged particles ( electrons ). This theory is known as the plum pudding model.
Ernest Rutherford
In 1909, Ernest Rutherford and two of his students conducted the now-infamous gold foil experiment. They fired positively charged start particles at a very thin sheet of gold. If the plum pudding model were correct, the particles would either pass through the sheet of gold or be very slightly deflected as the tuition was thought to be spread through the atom. Gold was chosen as it can be made very thin.
While most particles did pass through, some were deflected increasingly than Rutherford expected, and some were deflected backwards, showing that the plum pudding model could not be correct. Rutherford ripened a theory where the whit had a tiny positively charged nucleus in the centre with a deject of negative electrons surrounding it.
Alpha particles fired towards the gold foil were either deflected backwards if they were tropical to the nucleus or passed through the empty space of the atom.

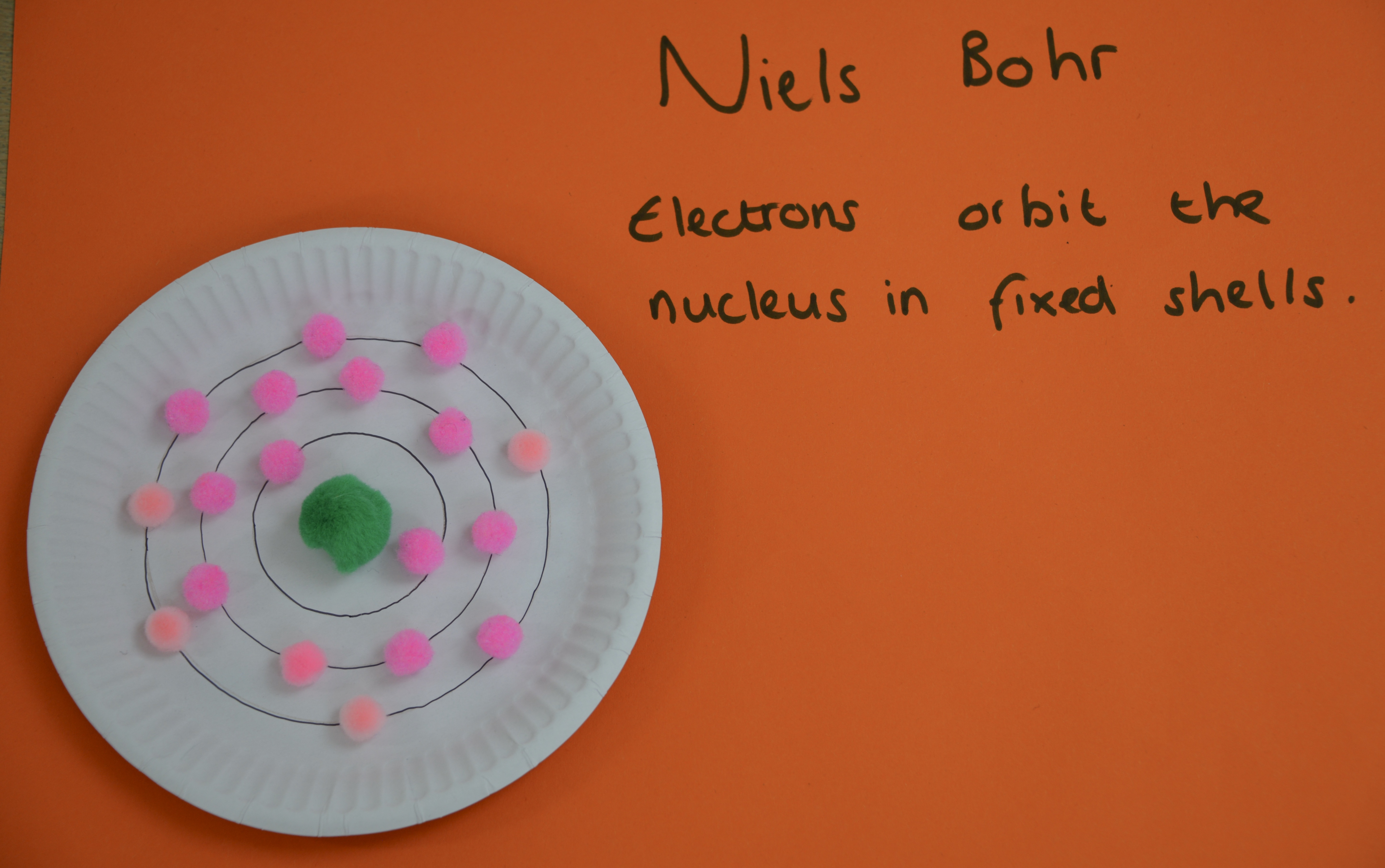
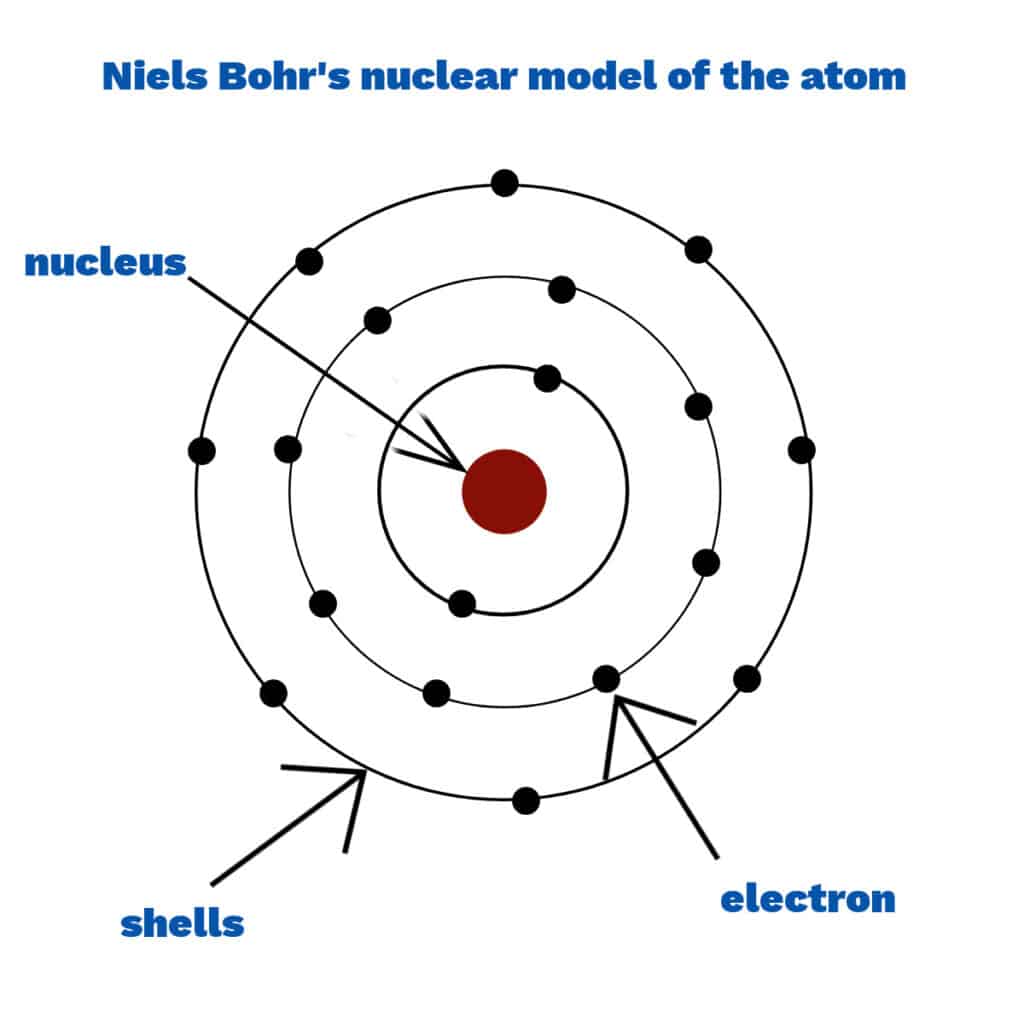
Niels Bohr
Niels Bohr ripened a model of the whit with electrons serried in stock-still shells virtually the nucleus instead of in a cloud. Scientists thought that electrons in a deject would be attracted to the nucleus, making the whit collapse.
James Chadwick
James Chadwick conducted experiments demonstrating that atoms have neutral particles ( neutrons ) in their nucleus. James Chadwick’s model is very tropical to the modern-day nuclear model of the atom!




Learn increasingly well-nigh the history of the atom
Learn increasingly well-nigh Ernest Rutherford, who was awarded a Nobel Prize for his work on two-bit structure.
Discover how Ernest Schrödinger extended the model proposed by Bohr.
Recommended For You
-
 How Fintech Innovations Changed My Financial LifeLet me tell you something exciting—I utilized to think cash...
How Fintech Innovations Changed My Financial LifeLet me tell you something exciting—I utilized to think cash... -
 Low Investment High Return Business Ideas That WorkStarting a business doesn’t always mean spending a lot of...
Low Investment High Return Business Ideas That WorkStarting a business doesn’t always mean spending a lot of... -
 The World of Data Science: What I Learned and How You Can TooI still keep in mind the day I to begin...
The World of Data Science: What I Learned and How You Can TooI still keep in mind the day I to begin... -
 Best Internet Security Software 2025: A Simple Guide Everyone Can TrustInternet security is a subset of computer security that includes...
Best Internet Security Software 2025: A Simple Guide Everyone Can TrustInternet security is a subset of computer security that includes...